ISABEL RUBIO ARROYO | Tungsteno
The ozone layer in the stratosphere serves as a vital protective shield for all life on Earth. This invisible barrier protects the inhabitants of our planet from the Sun’s harmful ultraviolet (UV) radiation, which can cause sunburn and damage the health of humans, as well as having other harmful effects on animals, plants and even microbes. But there's a problem: this layer has an enormous "hole"—a less dense region of the ozone layer that lets UV rays pass through. Fortunately, a new analysis by a UN-backed panel of experts offers some encouraging news. The global phaseout of ozone-depleting chemicals is helping to slowly close this hole.
The big threats to the ozone layer
To understand why the ozone hole is closing, we need to consider how it was formed. The ozone layer is a region of high ozone concentration in the stratosphere, 15 to 35 kilometres above the Earth's surface, according to the United Nations Environment Programme (UNEP). In the mid-1970s, scientists realised that it was threatened by the accumulation of gases containing halogens (chlorine and bromine) in the atmosphere.
These chemicals, collectively known as ozone-depleting substances (ODS), were found in many products and appliances that millions of people around the world used in their daily lives. For example, ODS were used in air conditioners, refrigerators, aerosol cans, inhalers for asthma patients, solvents to clean electronics, insulation foams in homes and office buildings, water boilers and even the soles of shoes.
Various chemical substances deplete the ozone layer. Credit: NASA Goddard
How did this enormous hole appear?
Research published in the scientific journal Nature in the mid-1980s warned that the concentration of stratospheric ozone over Antarctica was rapidly decreasing. This is known as the ozone hole, which in September 2000 reached a record 28.4 million square kilometres, according to the European Environment Agency. That is equivalent to almost seven times the size of the European Union.
But how were these chemicals destroying the ozone layer? When a chlorofluorocarbon molecule—the most damaging ODS—reaches the stratosphere, it eventually absorbs ultraviolet radiation, causing it to break down and release its chlorine atoms. The UNEP explains that "one chlorine atom can destroy up to 100,000 ozone molecules." When there are too many of these chlorine and bromine reactions, it disrupts "the delicate chemical balance that maintains the ozone layer, causing ozone to be destroyed faster than it is created."
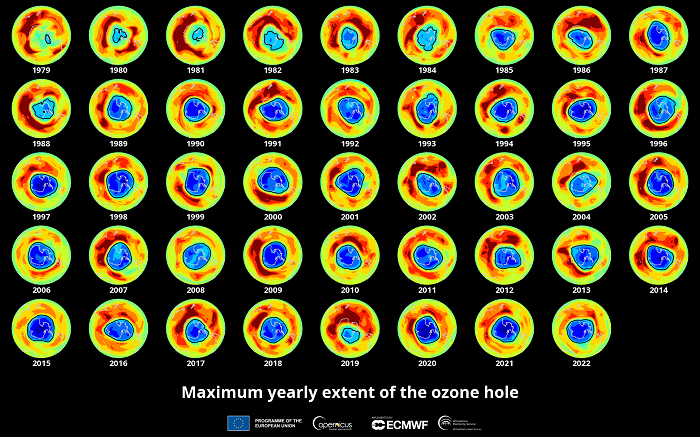
The ozone hole reached a record 28.4 million square kilometres in 2000.
The ambitious challenge of protecting this invisible shield
To prevent the hole from continuing to grow out of control, the Montreal Protocol was signed in 1987. This global agreement, which came into force in 1989, decreed the elimination of 96 ozone-depleting chemicals and brought about changes in the manufacturing processes of many products. While more than 800,000 tonnes of chlorofluorocarbons were consumed in 1989, by 2014 this figure had fallen to 156 tonnes, according to the World Economic Forum. Today, the UNEP notes that 99% of the ozone-depleting substances controlled by the Montreal Protocol have been phased out.
This has resulted in a slow recovery of the ozone layer. By the end of 2022, the hole had an average area of 23.2 million square kilometres, slightly down from 23.3 million the previous year and well below the 27.5 million reached in September 2006 when the average area of the hole peaked, according to the US National Oceanic and Atmospheric Administration (NOAA). The European Space Agency (ESA) explains that large fluctuations in polar vortices and stratospheric temperatures cause ozone holes to vary in size.
A ban on various chemicals is helping to close the ozone hole. Credit: BBC News
Political action and climate change
"Today, the ozone hole is recovering thanks to clear political action. This example shall serve as an inspiration for climate change," says Josef Aschbacher, ESA's Director of Earth Observation Programmes. However, while ozone-depleting substances are no longer being produced, more and more greenhouse gases are being emitted.
Without the Montreal Protocol, ozone depletion would have continued to spread across the globe, which could have affected the health of the planet's inhabitants, as the UNEP highlights. For example, according to the agency, a global model suggests that by 2030 the successful implementation of this agreement will be preventing about two million skin cancers each year.
If efforts continue, the UNEP predicts that the ozone layer will recover during this century: "If current policies remain in place, the ozone layer is expected to recover to 1980 values (before the appearance of the ozone hole) by around 2066 over the Antarctic, by 2045 over the Arctic and by 2040 for the rest of the world."
· — —
Tungsteno is a journalism laboratory to scan the essence of innovation.